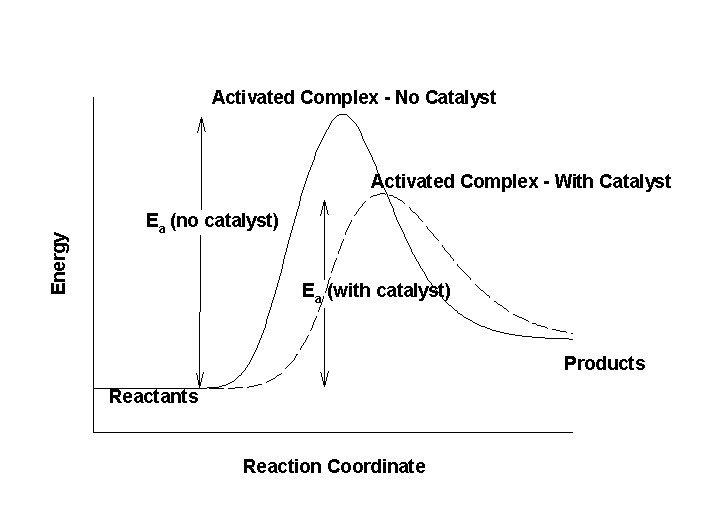
Are Catalysts Included In Rate Law . catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. It is possible for a catalyst to change the order of a reaction since it shows different path to the. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. use rate laws to calculate reaction rates. we do know that the concentration of a catalyst might affect the rate of reaction. But what if the catalyst is. Use rate and concentration data to identify reaction orders and derive rate laws.
from www.chm.uri.edu
yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. we do know that the concentration of a catalyst might affect the rate of reaction. But what if the catalyst is. use rate laws to calculate reaction rates. catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. Use rate and concentration data to identify reaction orders and derive rate laws. It is possible for a catalyst to change the order of a reaction since it shows different path to the. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law.
CHM 112 Lecture 6
Are Catalysts Included In Rate Law catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. But what if the catalyst is. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. Use rate and concentration data to identify reaction orders and derive rate laws. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. use rate laws to calculate reaction rates. we do know that the concentration of a catalyst might affect the rate of reaction. It is possible for a catalyst to change the order of a reaction since it shows different path to the.
From www.youtube.com
Practice Identifying Catalysts and Intermediates YouTube Are Catalysts Included In Rate Law catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. use rate laws to calculate reaction rates. It is possible for a catalyst to change the order of a reaction since it. Are Catalysts Included In Rate Law.
From www.slideserve.com
PPT Section 14.6 Reaction Mechanisms and Catalysis PowerPoint Are Catalysts Included In Rate Law catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. Use rate and concentration data to identify reaction orders and derive rate laws. It is possible for a catalyst to change the order. Are Catalysts Included In Rate Law.
From www.slideserve.com
PPT Reaction Mechanisms PowerPoint Presentation, free download ID Are Catalysts Included In Rate Law use rate laws to calculate reaction rates. catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. Use rate and concentration data to identify reaction orders and derive rate laws. yes,. Are Catalysts Included In Rate Law.
From www.scribd.com
of Chemical Reactions Rate Laws and Reaction Orders PDF Are Catalysts Included In Rate Law Use rate and concentration data to identify reaction orders and derive rate laws. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. But what if the catalyst is. It. Are Catalysts Included In Rate Law.
From slideplayer.com
Dr. Namphol Sinkaset Chem 201 General Chemistry II ppt download Are Catalysts Included In Rate Law the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. It is possible for a catalyst to change the order of a reaction since it shows different path to the. But what if the catalyst is. Use rate and concentration data to identify reaction orders and derive rate laws. . Are Catalysts Included In Rate Law.
From www.youtube.com
Catalysis 6 Synthesizing Rate Laws and mechanisms YouTube Are Catalysts Included In Rate Law the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders and derive rate. Are Catalysts Included In Rate Law.
From www.youtube.com
Integrated Rate Equation for first Order Reaction First Order Are Catalysts Included In Rate Law we do know that the concentration of a catalyst might affect the rate of reaction. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. Use rate and concentration data to identify reaction orders and derive rate laws. catalysts, which do not appear in the balanced overall chemical. Are Catalysts Included In Rate Law.
From www.youtube.com
AP Chemistry Unit 5 Review Reaction Rates Rate Law Are Catalysts Included In Rate Law use rate laws to calculate reaction rates. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. It is possible for a catalyst to change the order of a reaction since it shows different path to the. catalysts, which do not appear in the balanced overall chemical equation,. Are Catalysts Included In Rate Law.
From dokumen.tips
(PPTX) Chpt 12 Chemical Reaction Rates Rate Laws Reaction Are Catalysts Included In Rate Law we do know that the concentration of a catalyst might affect the rate of reaction. catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. use rate laws to calculate reaction rates. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate. Are Catalysts Included In Rate Law.
From www.researchgate.net
Origin of the rate law of heterogeneous catalysis from the mechanism of Are Catalysts Included In Rate Law we do know that the concentration of a catalyst might affect the rate of reaction. catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. Use rate and concentration data to identify reaction orders and derive rate laws. But what if the catalyst is. the relation between the rate of a. Are Catalysts Included In Rate Law.
From www.slideserve.com
PPT Reaction mechanisms and catalysts PowerPoint Presentation, free Are Catalysts Included In Rate Law yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. But what if the catalyst is. Use rate and concentration data to identify reaction orders and derive rate laws. catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. the relation between. Are Catalysts Included In Rate Law.
From www.youtube.com
Identifying catalysts in a reaction YouTube Are Catalysts Included In Rate Law we do know that the concentration of a catalyst might affect the rate of reaction. use rate laws to calculate reaction rates. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction. Are Catalysts Included In Rate Law.
From www.youtube.com
Rate Laws and the Method of Initial Rates YouTube Are Catalysts Included In Rate Law yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. we do know that the concentration of a catalyst might affect the rate of reaction. use rate laws to calculate reaction rates. It is possible for a catalyst to change the order of a reaction since it shows. Are Catalysts Included In Rate Law.
From www.studypool.com
SOLUTION Gen chem i collision theory reaction rates laws Are Catalysts Included In Rate Law catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. But what if the catalyst is. Use rate and concentration data to identify reaction orders and derive rate laws. we do know. Are Catalysts Included In Rate Law.
From www.nagwa.com
Question Video Describing the Effect of Catalysts on Reaction Rates Are Catalysts Included In Rate Law we do know that the concentration of a catalyst might affect the rate of reaction. catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. Use rate and concentration data to identify reaction orders and derive rate laws. yes, catalysts play a crucial role in determining reaction rates, and they are. Are Catalysts Included In Rate Law.
From slideplayer.com
Chemical ppt download Are Catalysts Included In Rate Law the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. But what if the catalyst is. Use rate and concentration data to identify reaction orders and derive rate laws. . Are Catalysts Included In Rate Law.
From www.youtube.com
Determining Rate Laws from Experimental Data YouTube Are Catalysts Included In Rate Law It is possible for a catalyst to change the order of a reaction since it shows different path to the. catalysts, which do not appear in the balanced overall chemical equation, can also influence reaction rate. the relation between the rate of a reaction and the concentrations of reactants is expressed by its rate law. use rate. Are Catalysts Included In Rate Law.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction Are Catalysts Included In Rate Law Use rate and concentration data to identify reaction orders and derive rate laws. yes, catalysts play a crucial role in determining reaction rates, and they are often included in the rate law. But what if the catalyst is. use rate laws to calculate reaction rates. the relation between the rate of a reaction and the concentrations of. Are Catalysts Included In Rate Law.